Cold molecules on collision course
Using a new cooling technique MPQ scientists succeed at observing collisions in a dense beam of cold and slow dipolar molecules.
How do chemical reactions proceed at extremely low temperatures? The answer requires the investigation of molecular samples that are cold, dense, and slow at the same time. Scientists around Dr. Martin Zeppenfeld from the Quantum Dynamics Division of Prof. Gerhard Rempe at the Max Planck Institute of Quantum Optics in Garching have now taken an important step in this direction by developing a new cooling method: the so-called “cryofuge” combines cryogenic buffer-gas cooling with a special kind of centrifuge in which rotating electric fields decelerate the precooled molecules down to velocities of less than 20 metres per second.
Due to the high flux densities that were attained the team succeeded in observing collisions between the cold molecules. For two chemical compounds with a strong electric dipole moment, the collision probability as well as its dependence on velocity and flux density was thereby determined (Science, 13 October 2017). The new technique is a milestone for the emerging field of cold chemistry and could open the perspective towards controlling and manipulating chemical pathways at extremely low temperatures.

The production of cold molecules has proven to be a big challenge: laser-cooling – a very efficient method for atoms – in general doesn’t work for molecules because they exhibit vibrational and rotational states in addition to the electronic states. On the other hand, a large number of molecules, e.g. water (H2O), possess an uneven electric charge distribution. Molecules with such an electric dipole moment can be influenced and hence decelerated by electric fields.
The MPQ team has mostly experimented with fluoromethane (CH3F) and deuterated ammonia (ND3). Initially, the molecules have a temperature of around 200 Kelvin and a velocity of several hundred metres per second. As a first step, the molecules thermalize with a helium or neon buffer-gas in the cryogenic buffer-gas cell and get cooled down to 6 Kelvin (helium) and 17 Kelvin (neon) respectively. They are extracted from the cryogenic environment by a bent electrostatic quadrupole guide. When they exit the buffer-gas cell, their speed has been reduced to 50 to 100 metres per second. “However, it is not only the velocity that matters”, emphasizes Dr. Martin Zeppenfeld, leader of the project. “Regarding the molecular collisions that we aim to observe it is crucial that during this cooling process also the internal states are being cooled. We can prove that only very few and low rotational and vibrational states are excited.”
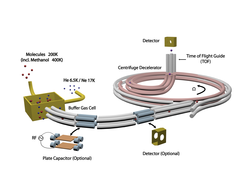
By a straight guide the molecules are transferred to the second part of the cooling device, the centrifuge decelerator. “Varying the guiding voltage on the straight guide we can control the trap depth and thereby the molecular beam densities,” explains Thomas Gantner, doctoral candidate at the experiment. “The higher the voltage, the higher the beam density. This kind of control is necessary in order to get a better understanding of the mechanisms behind the cold dipolar collisions that we are going to measure after the deceleration process.”
Entering the centrifuge, the molecules first propagate around the periphery in a stationary storage ring with a diameter of 40 centimetres composed of two static and two rotating electrodes. Then a rotating electric quadrupole guide picks up the molecules almost at any point around the storage ring and pushes them along its spiral shape towards the rotation axis. Thus, while the electric fields make the molecules move into the centre of the disc, they constantly have to counteract the centrifugal force induced by the quadrupole guide that rotates at 30 Hertz, thereby continuously slowing the molecules down.
A final straight guide brings the molecules to a quadrupole mass spectrometer where they are analysed regarding their velocity. “The molecules spend about 25 milliseconds inside the quadrupole guide,” says Thomas Gantner. “This is plenty of time for them to interact, and in these collisions, molecules are being lost. The analysis reveals that the losses increase for decreasing velocities and increasing beam densities. The evaluation of the data relies to a large extent on model calculations that were done by Xing Wu, who is first author of this work and achieved his doctoral degree on this experiment.”
“The observation of cold molecular collisions is a milestone for the field of cold chemistry,” emphasizes Dr. Zeppenfeld. “The generic principle underlying the cryofuge enables its application to a wide range of dipolar compounds. We envision the possibility that in the future chemical reactions with long interaction times can be realized at very low temperatures.”
Furthermore, the cryofuge could extend the range of research topics that experiments with cold molecules offer. For instance, the cold and slow beam of methanol produced could be ideally suited for measuring variations in the proton-to-electron mass ratio. According to theoretical predictions these could be caused by interaction with dark matter. The cryofuge could also serve as a perfect source for ongoing experiments with laser-coolable diatomic molecules. On the other hand, the long-range and anisotropic dipole coupling mediates interactions over micrometre distances. This renders cold polar molecules particularly suitable for applications in quantum simulation or quantum computing. “The very first observation of collisions in a cold gas of naturally occurring molecules brings us closer to the dream of achieving a complex quantum gas such as a Bose Einstein condensate of water molecules,” says Prof. Gerhard Rempe. Olivia Meyer-Streng













